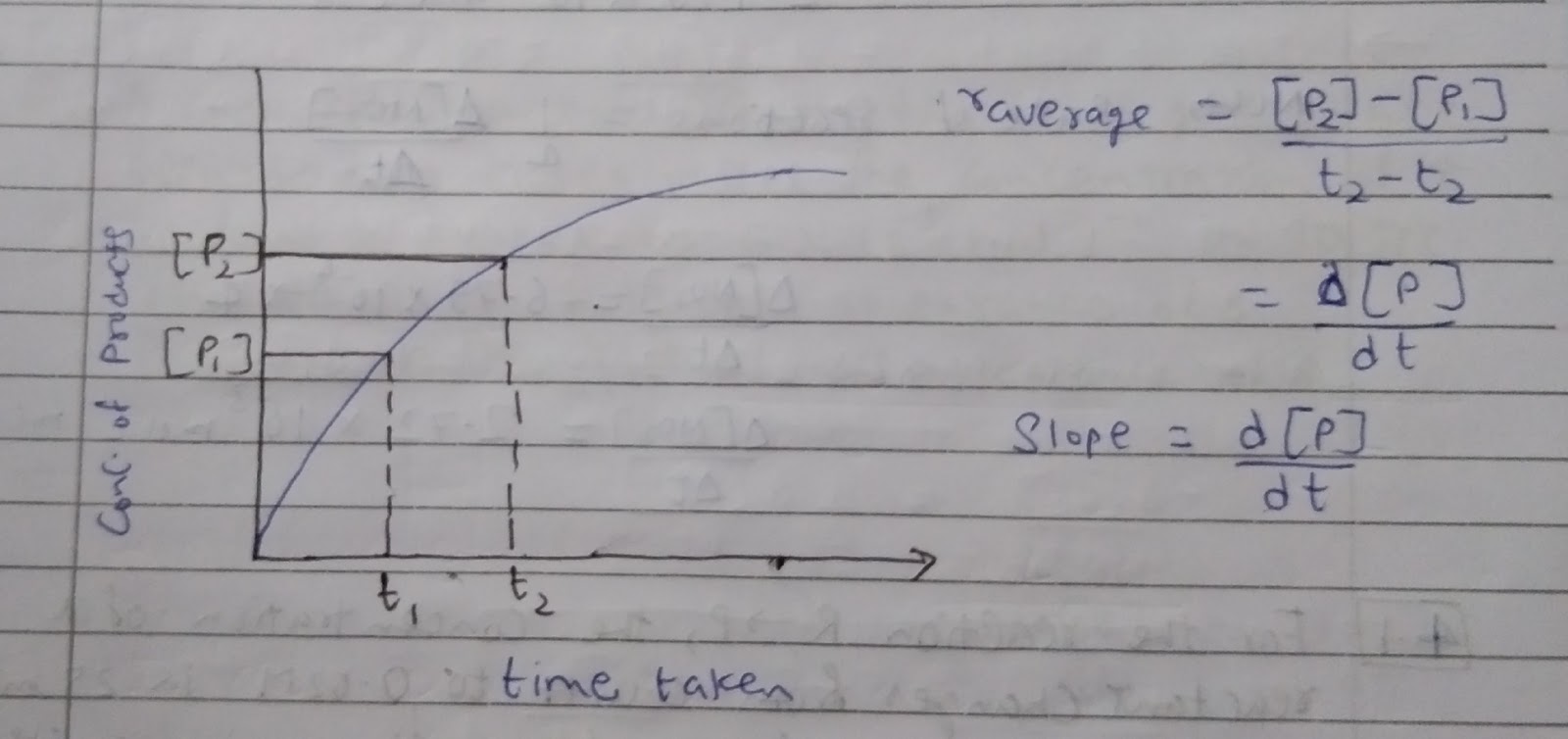
Average Rate of reaction
Average rate of reaction depends on change in concentration of reactants or products and the time taken for that change.
Rate Law
Rate law gives the rate of reaction and the concentration of reactant.
Rate law is expression in which reaction rate is given in terms of molar concentration of reactant with each term rise to some Power which may or may not be same as the stoichiometric coefficient of the reacting species in the balanced chemical equation.
Zero order reaction
Graphical representation of zero order reaction
Example of zero order reaction
First order reaction
Graphical representation of first order reaction




















0 Comments